TECHNOLOGY
Catalyst technology to save the Earth and people
What is a Catalyst?

Definition of catalyst
What comes to your mind when you hear the word ‘catalyst’?
If you’re interested in cars, you might have thought of emissions reduction devices, or if you’re interested in life sciences, you might have thought of enzymes.
In addition, some may have thought of a hot pack that melts the hands and feet of wonderful soldiers who are fulfilling their national defense obligations on a cold winter day.
Then let’s look at ‘Participating in a chemical reaction’. A chemical reaction is a phenomenon in which one substance is converted into another.
In more detail, the movement of electrons at the molecular level causes a change in molecular structure.
Therefore, participating in a ‘chemical reaction’ means exchanging electrons at the molecular level, and differently put, it means a change in chemical bonds.
A chemical bond here is a bond that acts between its members in a stable atom or group of atoms, such as a molecule.
For example, it converts harmful gases from automobiles to be harmless to the human body.
The most widely used catalyst is rhodium palladium platinum, a platinum group, which has recently seen a surge in demand and a sharp rise in prices due to poor mining.
Furthermore, since it operates only at high temperatures, it is not possible to purify the fumes in the low-temperature Saatae early in the start-up.

Then, is there any metal that can be used as a catalyst at low temperatures while providing a stable supply?
I have it.
In 1987, there was a groundbreaking report that the use of gold nanoparticles could lead to catalytic reactions at room temperature.
Its performance is so good that the cost of raw materials can be reduced by less than a tenth compared to conventional platinum group catalysts, which also ensures high economic efficiency.
So why didn’t they use gold nanocatalysts before?
Gold nanocatalysts had such great performance that they could not be commercialized until now due to the disadvantage of particles clumping together so easily that they became inactive.

But quantum cats trap each gold nanoparticle in a nanocage.
By fundamentally blocking the phenomenon of clumping together, it enabled the commercialization of gold nanocatalyst for the first time in the world.

Quantum Cat catalysts can reduce car fuel consumption and harmful gases at the same time, and purify emissions from power plants and factories at a much lower cost, making the Earth’s air cleaner.
Also, organic compounds dissolved in water are easily oxidized at room temperature to remove odors from the water and to drink healthy water.
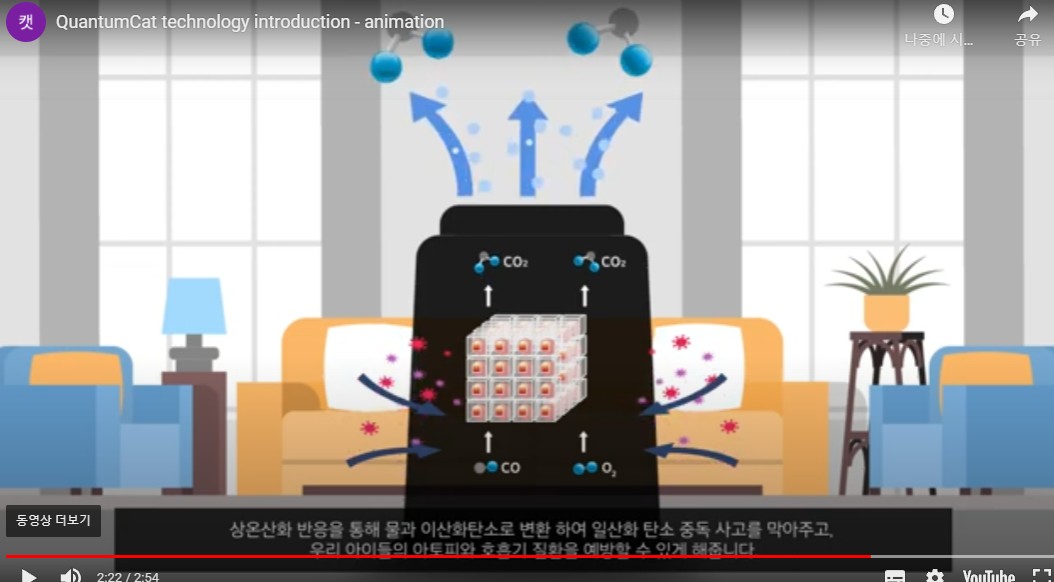
At home, harmful gases such as carbon monoxide and volatile organic compounds are converted into water and carbon dioxide through room temperature oxidation reactions to prevent carbon monoxide poisoning accidents and prevent atopic and respiratory diseases in our children.
Quantum cat catalysts can easily oxidize hydrogen at room temperature.
These capabilities can be used to prepare for the upcoming era of hydrogen economy more safely, increase energy efficiency by more than 30% compared to conventional fuel cells, and dramatically extend fuel cell life.